
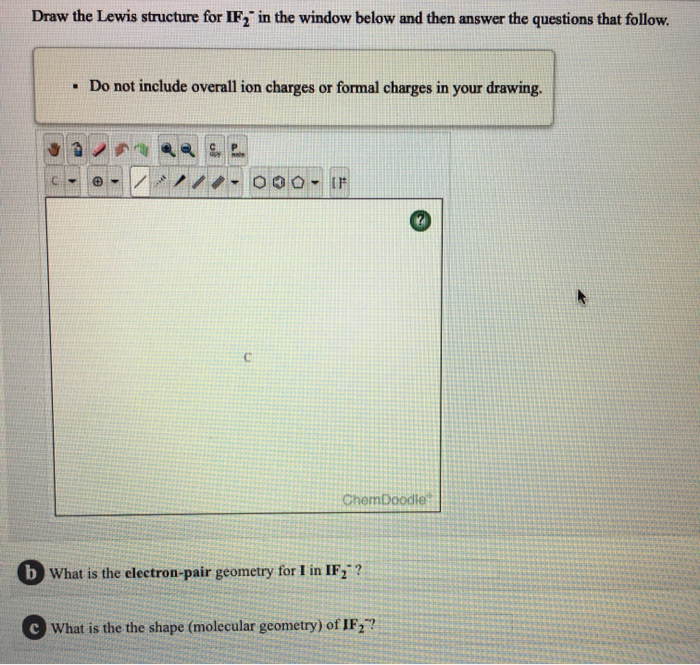
IF2 Lewis structure, Molecular geometry, and Polar or nonpolar
this is the complete Lewis structure of CO 2. For Lewis structure purposes, the lone-pairs can only be moved from terminal atoms to the central atom to form multiple bonds, not the other way around. 7. Formal charges check: all atoms have formal charges equals to 0 in this structure. FC (C) = 4 -½× (4×2) = 0.

How to Draw the Lewis Dot Structure for IF2 + YouTube
An explanation of the molecular geometry for the IF2 - ion (Iodine difluoride anion) including a description of the IF2 - bond angles. The electron geometry.

Geometry Of Molecules IF2 Lewis Structure How to Draw the Lewis
The Lewis structure of H 2 O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds: We predict that these four regions are arranged in a tetrahedral fashion (Figure 7.23), as indicated in Figure 7.19. Thus, the electron-pair geometry is tetrahedral and the molecular.

SOLVED Predict the electron pair geometry of IF2. (Hint Draw a
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 6.4.1 6.4. 1 shows the Lewis symbols for the elements of the third period of the periodic table. Electron dots are typically arranged in four pairs located on the four "sides" of the atomic symbol.

Iodine Dot Diagram exatin.info
In if2- lewis structure we see that each F atom has 8 valance electrons and completes their octet. In I forms 2 I-F bonds and each bond contain 2 electrons. There is also 3 lone pairs that present on I atom making a total of 10 electrons around I atom.

IF2 Lewis Structure How to Draw the Lewis Structure for IF 2 YouTube
This is the IF2- Lewis structure. Iodine, 7 valence electrons. Fluorine also has 7, but we have two Fluorines; and then we have an extra valence electron from that negative sign for a total of 22 valence electrons. Iodine's the least electronegative. Let's put that in the center and then Fluorines can go on the outside. We'll put two valence electrons between the atoms to form chemical bonds.

Iodine Dot Diagram exatin.info
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

IF2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
IF2- lewis structure has an Iodine atom (I) at the center which is surrounded by two Fluorine atoms (F). There is a single bond between the Iodine atom (I) and each Fluorine atom (F). There is a -1 formal charge on the Iodine atom (I).

Answered Draw the Lewis structure of IF2O2¯… bartleby
In IF 2- Lewis structure, there are two single bonds around the iodine atom, with two fluorine atoms attached to it, and each atom has three lone pairs. Also, there is a negative (-1) charge on the iodine atom. IF2- Lewis Structure: How to Draw the Lewis Structure for IF2 - Watch on Contents Steps #1 Draw a rough skeleton structure
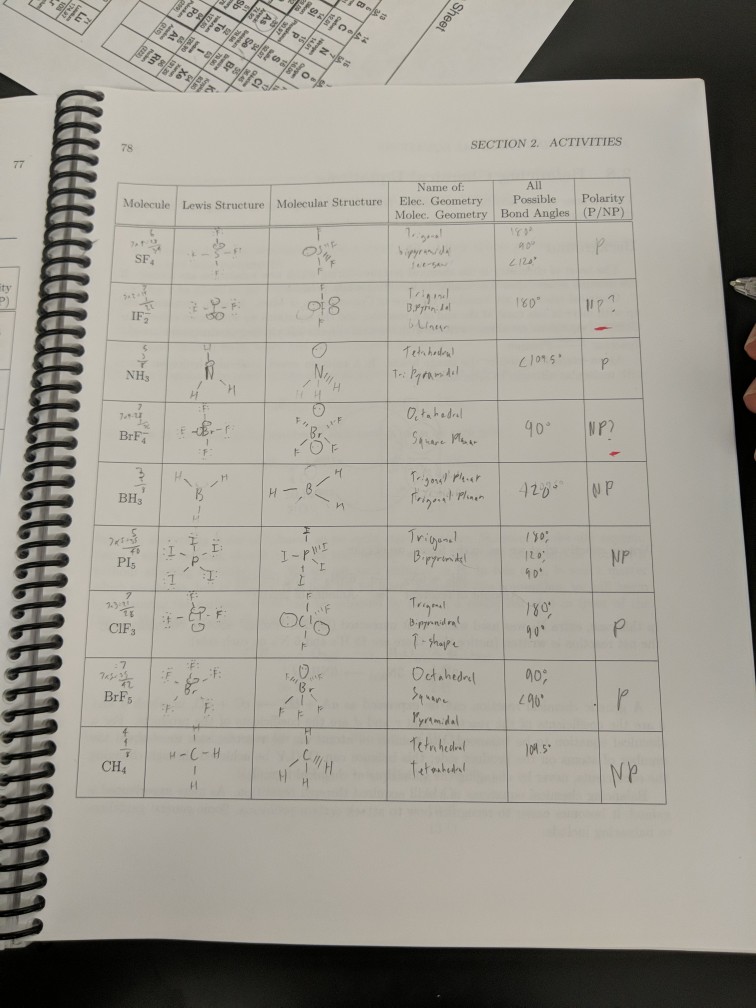
Solved SECTION 2. ACTIVITIES Molecule Lewis Structure
IF2- lewis structure contains one iodine atom at the middle position whereas two fluorine atoms at the surrounding position. There are three lone pairs present on the central atom of IF2- lewis structure. Also, the iodine central atom in IF2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell.

Iodine Dot Diagram exatin.info
The IF 2- Lewis structure you'll need to put more than eight valence electrons on the Iodine atome. In the Lewis structure for IF 2- there are a total of 22 valence electrons. Watch on See the Big List of Lewis Structures Transcript: This is the IF2- Lewis structure. Iodine, 7 valence electrons.

Iodine Dot Diagram exatin.info
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.
Solved Draw the Lewis structure for IF in the window below
IF2- Lewis Structure | How to Draw the Lewis Structure for IF2- - YouTube © 2023 Google LLC IF2- comprises one Iodine and two Fluorine atoms. The negative charge on this ion indicates.

(Get Answer) (ClO3; IF2; XeCl2F2) 2) (CN; CN+; CN) 3) Draw the
IF2- comprises one Iodine and two Fluorine atoms. The negative charge on this ion indicates that it accepts an additional electron to form this structure. Generally, the ions that accept electrons acquire a negative charge, whereas those that donate electrons acquire a positive charge.

PPT Ch. 10a Chemical Bonding II Molecular Shapes PowerPoint
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
What is the Lewis structure for IF2? YouTube
A step-by-step explanation of how to draw the IF2- Lewis Structure. For the IF2- structure use the periodic table to find the total number of valence electrons for.more.more A.